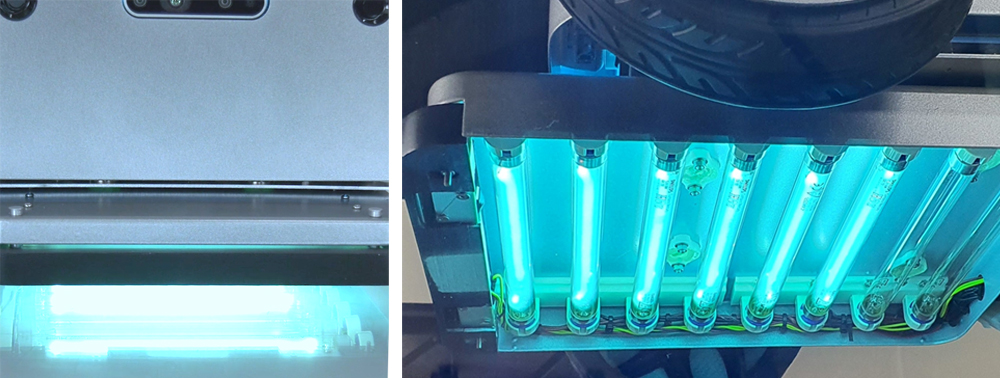
UVC sterilization mission module design and production
- · UVC sterilization module design technology according to sterilization target
- Spatial structure and disinfection target area
- Module design according to sterilization time
- Technologies to Prevent UVC Exposure
- · Optimal UVC module design technology for the purpose
- Optimal output calculation, required lamp and LED specification design technology
- Calculation of sterilization time based on target sterilization power
- Technologies to Prevent UVC Exposure
- · Fabrication of UVC lamp driving circuit for mobile robot
UVC
- · It is a high-energy light with a wavelength of less than 280 nm and works to change the structure of DNA and RNA in cells.
- · Pathogenic viruses, bacteria, and protozoa all have DNA and RNA, and UVC causes structural changes in DNA and RNA to inactivate viruses and sterilize them.
UVC sterilization effect
- · The UVC sterilization effect is determined by the amount of UVC light intensity integrated during the irradiation time. Accumulated light amount = light intensity x irradiation time
- · In the case of Rotavirus SA-11, 9.1 mJ/cm2 for 90% sterilization and 48 mJ/cm2 for 99.999% sterilization. It can kill 90% of all rotavirus within 1 square centimeter in time, and it takes about 5 seconds to kill 99.999%.
However, the amount of UV light is inversely proportional to the square of the distance, and there is an amount of shaded or reflected light, so the actual amount of UVC applied can vary greatly depending on the design of the sterilization module.
Table picture


Source : Chevrefils, Gabriel, et al. "UV dose required to achieve incremental log inactivation of bacteria, protozoa and viruses." IUVA News 8.1 (2006): 38-45.
※ References related to UVC sterilization effect
Sommer, R., Pribil, W., Appelt, S., Gehringer, P., Eschweiler, H., Leth, H., Cabaj, A. and Haider, T. 2001. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: A comparative approach, Wat. Res., 35(13): 3109- 3116.
Lazarova,V. and Savoye, P. 2004. Technical and sanitary aspect of wastewater disinfection by ultraviolet irradiation for landscape irrigation, Wat. Sci. Technol., 50(2): 203-209.
Sommer, R., Weber, G., Cabaj, A., Wekerle, J., Keck, G., and Schauberger, G. 1989. UV inactivation of microorganisms in water. Zbl. Hyg. 189: 214-224.
Husman, A.M.D., Bijkerk, P., Lodder, W., Van den Berg, H., Pribil, W., Cabaj, A., Gehringer, P., Sommer, R. and Duizer, E. 2004. Calicivirus inactivation by nonionizing 253.7-nanometer-wavelength (UV) and ionizing (Gamma) radiation, Appl. Environ. Microbiol., 70(9): 5089-5093.
Thurston-Enriquez, J.A. , Haas, C.N. , Jacangelo, J. , Riley, K. and Gerba, C.P. 2003. Inactivation of feline calcivirus and adenovirus type 40 by UV radiation, Appl. Environ. Microbiol., 69(1): 577-582.
Gerba, C.P., Gramos, D.M. and Nwachuku, N. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light, Appl. Environ. Microbiol., 68(10): 5167-5169.
Thompson, S.S., Jackson, J.L., Suva-Castillo, M., Yanko, W.A., Jack, Z.E., Kuo, J., Chen, C.L., Williams, F.P. and Schnurr, D.P. 2003. Detection of infectious human adenoviruses in tertiary-treated and ultraviolet-disinfected wastewater, Wat. Environ. Res., 75(2): 163-170.
?Meng, Q.S. and Gerba, C.P. 1996. Comparative inactivation of enteric adenoviruses, poliovirus and coliphages by ultraviolet irradiation, Wat. Res., 30(11):2665-2668.
Wilson, B.R., Roessler, P.F., Van Dellen, E., Abbaszadegan, M. and Gerba, C.P. 1992. Coliphage MS-2 as a UV water disinfection efficacy test surrogate for bacterial and viral pathogens, Proceedings, Water Quality Technology Conference, Nov 15-19, 1992, Toronto, Canada, pp. 219-235, Amer. Wat. Works Assoc., Denver, CO.
Liltved, H. and Landfald, B. 1996. Influence of liquid holding recovery and photoreactivation on survival of ultraviolet-irradiated fish pathogenic bacteria, Wat. Res., 30(5): 1109-1114.
Giese, N. and Darby, J. 2000. Sensitivity of microorganisms to different wavelengths of UV light: implications on modeling of medium pressure UV systems, Wat. Res., 34(16): 4007-4013.
Hoyer, O. 1998. Testing performance and monitoring of UV systems for drinking water disinfection, Wat. Supply, 16(1-2): 424-429.
Wu, Y., Clevenger, T. and Deng, B. 2005. Impacts of goethite particles on UV disinfection of drinking water, Appl. Environ. Microbiol., 71(7): 4140-4143.
Sommer, R., Haider, T., Cabaj, A., Pribil, W. and Lhotsky, M. 1998. Time dose reciprocity in UV disinfection of water, Water Sci. Technol., 38(12): 145-150.
Otaki, M., Okuda, A., Tajima, K., Iwasaki, T., Kinoshita, S. and Ohgaki, S. 2003. Inactivation differences of microorganisms by low pressure UV and pulsed xenon lamps, Wat. Sci. Technol., 47(3): 185-190.
Tosa, K. and Hirata, T. 1999. Photoreactivation of enterohemorrhagic E. coli following UV disinfection, Wat. Res., 33(2): 361-366.
Oguma, K., Katayama, H. and Ohgaki, S. 2004. Photoreactivation of Legionella pneumophila after inactivation by low- or medium-pressure ultraviolet lamp, Wat. Res., 38(11): 2757-2763.
Yaun, B.R., Sumner, S.S., Eifert, J.D. and Marcy, J.E. 2003. Response of Salmonella and E. coli O157:H7 to UV energy, J. Food Protection, 66(6): 1071-1073.
Chang, J.C.H., Osoff, S.F., Lobe, D.C., Dorfman, M.H., Dumais, C.M., Qualls, R.G. and Johnson, J.D. 1985. UV inactivation of pathogenic and indicator microorganisms, Appl. Environ. Microbiol., 49(6): 1361-1365.
Harris, G.D., Adams, V.D., Sorensen, D.L. and Curtis, M.S. 1987. Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria, Wat. Res., 21(6): 687-692.